
Entyce® (capromorelin oral solution)
Entyce is the only FDA-approved veterinary therapeutic for appetite stimulation in dogs.
Prescribe Entyce with confidence
 Entyce® delivers visible results, with ~7/10 dog owners reporting increased appetite in just 4 days of treatment compared to 4.5/10 control dogs.¹
Entyce® delivers visible results, with ~7/10 dog owners reporting increased appetite in just 4 days of treatment compared to 4.5/10 control dogs.¹ Dogs with long-term health conditions can safely take Entyce® throughout their treatment journey, even with a wide range of comorbidities.*
Dogs with long-term health conditions can safely take Entyce® throughout their treatment journey, even with a wide range of comorbidities.* Entyce works like the body’s own natural hunger hormone, ghrelin.
Entyce works like the body’s own natural hunger hormone, ghrelin. Entyce increases appetite, leading to increased food consumption.
Entyce increases appetite, leading to increased food consumption.
Entyce Product Information

For appetite stimulation in dogs

An oral solution

Mimics ghrelin, triggering dogs to eat

Safe to use daily for as long as it’s needed*
Entyce Dosing Information
Administer Entyce orally at a dose of 3 mg/kg (1.4 mg/lb) body weight once daily.
Available by prescription only, Entyce for dogs is supplied as a 30 mg/mL oral solution, includes a syringe for ease of administration, and is available in three bottle sizes: 10 mL, 15 mL, and 30 mL.
Entyce works like the body’s own natural hunger hormone, ghrelin
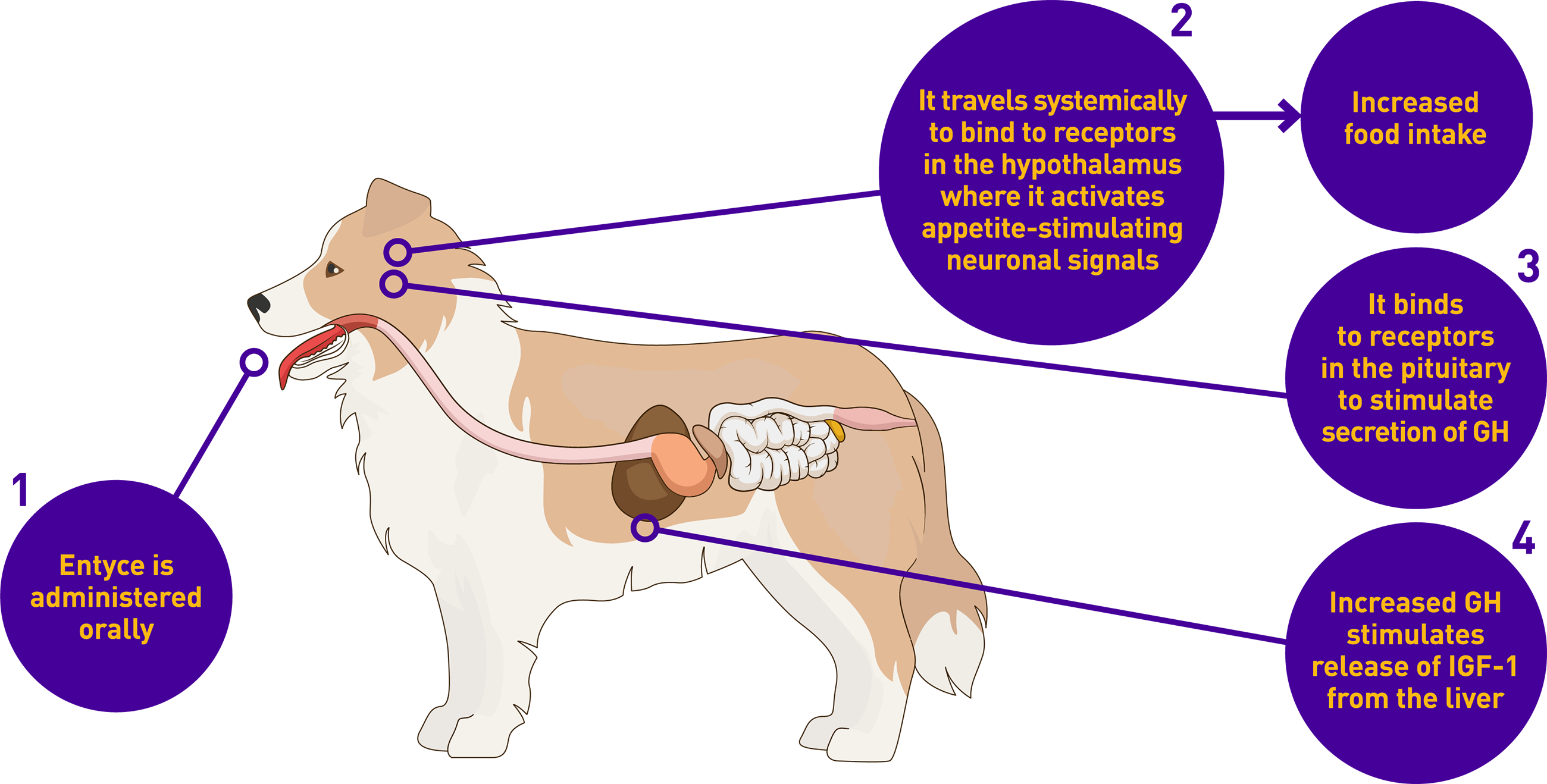
Entyce is safe to use daily, leading to changes pet owners can see
 Willingness to eat
Willingness to eat Anticipation of mealtime
Anticipation of mealtime Hunger behavior
Hunger behavior Enthusiasm for food
Enthusiasm for food
Consider Entyce as an essential part of your treatment plan
In the field study, 171 inappetent dogs were assessed for safety over 4 days1
- Dogs had a wide range of medical conditions.1
- Entyce was generally well tolerated.
The most commonly observed adverse events included diarrhea, vomiting, elevated blood urea nitrogen, polydipsia, and hypersalivation.
Toxicity was assessed for 12 consecutive months of treatment in healthy dogs, supporting safety at the labeled dose.2
Findings included increased salivation, reddening/swollen paws, increased liver weights, and hepatocellular cytoplasmic vacuolation.
More than a meal
Entyce supports dogs facing long-term health challenges by addressing inappetence, which is essential for improving nutrition.3
Entyce® (capromorelin oral solution) Resources

2021 Proceedings: Managing appetite disorders and weight loss in patients with chronic conditions
*The effectiveness of Entyce has not been studied beyond 4 days in the clinical study.
INDICATION: For appetite stimulation in dogs.
IMPORTANT SAFETY INFORMATION: For use in dogs only. Do not use in dogs that have a hypersensitivity to capromorelin. Use with caution in dogs with hepatic dysfunction or renal insufficiency. The safe use of Entyce has not been evaluated in breeding, pregnant or lactating dogs. The most common adverse reactions included diarrhea, vomiting, elevated blood urea nitrogen, polydipsia and hypersalivation.
Please see Entyce product label for full prescribing information.





